- Waist circumference reduced by up to 3.9 inches (10 cm), with sustained effects two weeks after treatment discontinuation
- Average half-life of 80 hours demonstrates potential for once-weekly dosing
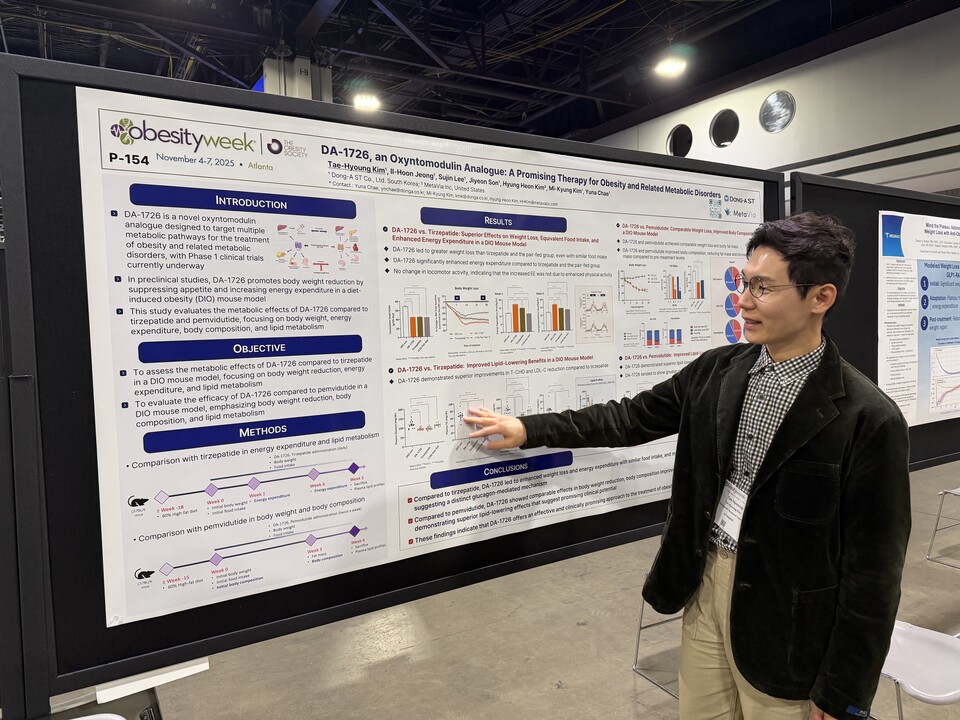
[by Ji, Yong Jun] Dong-A ST and its affiliate MetaVia announced on November 5 that they presented the results of a global Phase 1 clinical trial and new preclinical research findings for ‘DA-1726’ (development code), an investigational obesity treatment, in a poster session at the ObesityWeek 2025 conference organized by the Obesity Society, which opened on November 4 (local time) in Atlanta, Georgia, USA.
바카라 에볼루션1726 is a novel drug candidate in the ‘oxyntomodulin analog’ class currently in development as an anti-obesity drug candidate. It acts as a dual agonist of the ‘glucagon-like peptide 1 (GLP-1) receptor’ and the ‘glucagon receptor,’ suppressing appetite, stimulating insulin secretion, and enhancing basal metabolic rate in peripheral tissues. Ultimately, these mechanisms contribute to body weight loss reduction and glycemic control.
The Phase 1 clinical trial was conducted as a randomized, double-blind, placebo-controlled study involving nine obese adult participants to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of 바카라 에볼루션1726 at single and multiple doses. In this study, 바카라 에볼루션1726 was administered subcutaneously (SC) at a dose of 32 mg once weekly for four weeks, without dose titration.
The study results indicated that participants in the 바카라 에볼루션1726 group achieved a maximum weight loss of 6.3% (6.8 kg) and an average weight reduction of 4.3% (4.0 kg) after 26 days of treatment. Additionally, waist circumference decreased by up to 3.9 inches (10 cm), with the therapeutic effect persisting for two weeks following treatment completion. The study also demonstrated dose-linear pharmacokinetics and an average half-life of 80 hours, supporting the feasibility of once-weekly dosing.
The newly presented preclinical study was conducted using a high-fat diet-induced obesity (DIO) mouse model. 바카라 에볼루션1726 induced weight reduction through a combination of appetite suppression and increased energy expenditure. When compared with tirzepatide, 바카라 에볼루션1726 produced superior weight loss outcomes despite comparable food intake, attributed to a significantly greater increase in basal metabolic rate. This increase in energy expenditure remained substantial even in the absence of higher exercise activity. Furthermore, 바카라 에볼루션1726 achieved greater reductions in total cholesterol (T-CHO) and low-density lipoprotein cholesterol (LDL-C), suggesting a metabolic mechanism mediated by glucagon receptor activation.
Compared to pemvidutide, a drug belonging to the same therapeutic class, 바카라 에볼루션1726 exhibited comparable weight loss efficacy while achieving improved body composition, characterized by a reduction in fat mass and relative preservation of lean mass. In addition, it induced greater decreases in total cholesterol, LDL-C, and triglyceride levels, highlighting its superior lipid-lowering properties.
MetaVia has been conducting an additional Phase 1 clinical trial since July, designed to evaluate the maximum tolerated dose (MTD) of 바카라 에볼루션1726 over an eight-week treatment period at a 48 mg dose. The company plans to disclose the clinical data later this year, with expectations of confirming enhanced weight loss efficacy, as well as favorable safety and tolerability profiles.
"The Phase 1 clinical trial demonstrated excellent safety, along with early onset of weight loss, reduction in waist circumference, and cardiovascular safety. Moreover, the pharmacokinetic profile and 80-hour half-life of 바카라 에볼루션1726 underscore its potential as a once-weekly obesity treatment," said Kim Hyung-heon, CEO of MetaVia. "바카라 에볼루션1726 continues to strengthen its competitiveness as a differentiated obesity treatment, and we aim to further validate its clinical competitiveness through the ongoing Phase 1 clinical study assessing the maximum tolerated dose," he added.

