- [WCLC 2025] Interview with Chung Chul-woong, CTO and Head of Novel Drug Research
- "Precise tumor cell targeting with soluble 온라인 바카라 사이트 penetration"
- "Anticipated differentiation in solid tumors, including NSCLC and CRC"
- "Phase 1 clinical trial scheduled for launch in Q2 2027"
- "Rising global interest in 온라인 바카라 사이트 as expectations grow for overcoming ADC limitations after competing drugs’ clinical trial suspensions"
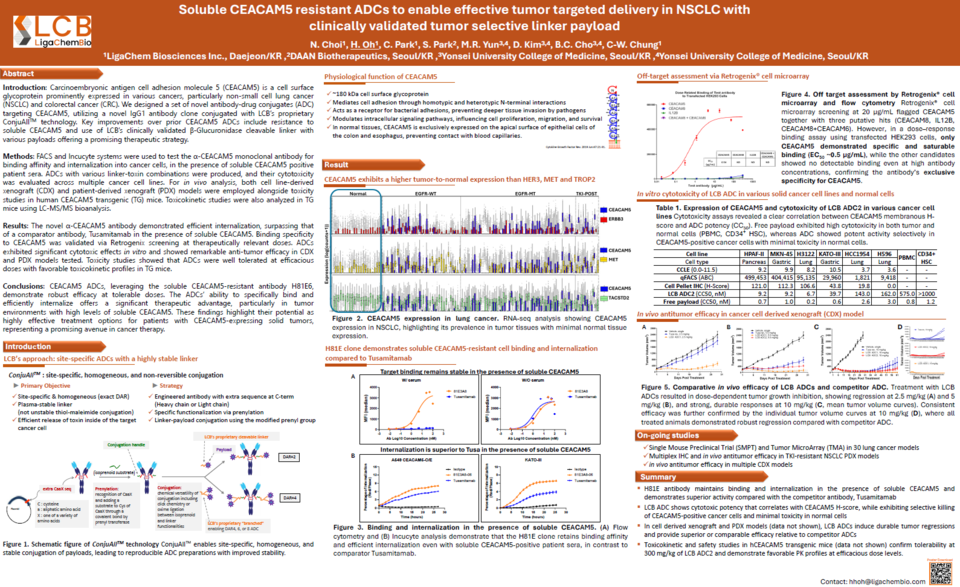
[by Lee, Young Sung] LigaChem Biosciences (hereafter referred to as LigaChem) has disclosed preclinical data on its novel antibody-drug conjugate (ADC), LCB58A (CEACAM5 ADC), targeting solid tumors, including non-small cell lung cancer (NSCLC) and colorectal cancer (CRC).
The data indicated superior tumor cell binding, enhanced tumor growth inhibition, and a favorable safety profile compared with competing drugs. As most rival drugs in the global market remain at early development stages, 온라인 바카라 사이트 aims to position LCB58A as a ‘best-in-class’ therapeutic. The company plans to initiate Phase 1 clinical trials in the second quarter of 2027.
These findings were presented as a poster at the World Congress on Lung Cancer (WCLC 2025) on September 8 (local time).
Chung Chul-woong, Head of 온라인 바카라 사이트 New Drug Research, stated in an interview with <THE BIO on September 8 (local time) during the poster presentation, "Although the program remains at an early stage of development, competitors visited the poster this morning and expressed interest."
◇LCB58A overcomes limitations of current 온라인 바카라 사이트 ADCs
온라인 바카라 사이트, the target of LCB58A, is a tumor marker expressed in non-small cell lung cancer and colorectal cancer and has garnered attention as a potential ADC development target. However, existing 온라인 바카라 사이트 ADCs have been criticized for their high binding affinity to ‘soluble (blood-dissolved) 온라인 바카라 사이트’ present in patient serum, which markedly reduces their internalization efficiency into tumor cells.
For instance, Sanofi's ‘Tusamitamab Ravtansine’ (development code SAR408701) demonstrated safety and preliminary efficacy in preclinical and Phase 1/2 studies but failed to meet endpoints in the Phase 3 (CARMEN-LC03) trial and was subsequently discontinued.
At present, Merck KGaA of Germany is conducting the most active early-stage clinical trials of a 온라인 바카라 사이트-targeting ADC with its candidate ‘Precem-TcT.’
"Sanofi discontinued Phase 3 trial due to the absence of a superior therapeutic index," Chung emphasized. "The critical factor is whether the ADC can reach tumor cells without leaking into the bloodstream and how efficiently it is internalized by the cells."
According to the company, LCB58A, developed by LigaChem, employs a novel IgG1 antibody clone (H81E6) that sustains stable tumor cell binding and internalization even under conditions of elevated blood-soluble CEACAM5 levels. In addition, the company's proprietary ConjuAll™ technology, combined with a clinically validated β-glucuronidase (β-Glu) cleavage linker, enables selective drug delivery to cancer cells.
The antibody was licensed from the Korean firm DAAN Biotherapeutics, founded by Cho Byoung-chul, Chief of the Lung Cancer Center at Yonsei Cancer Center. Cho is widely recognized as the Principal Investigator (PI) for Yuhan Corporation's non-small cell lung cancer treatment, Leclaza (lazertinib).
◇“Preclinical efficacy and safety demonstrated, surpassing the Limitations of Existing 온라인 바카라 사이트 ADCs”
The research team assessed antibody binding affinity and internalization using FACS and Incucyte systems and confirmed the anticancer activity of ADCs engineered with diverse linker-payload combinations.
As a result, LCB58A exhibited superior binding specificity and internalization compared with tusamitamab, even under conditions of soluble 온라인 바카라 사이트, and demonstrated robust antitumor efficacy in both CDX and PDX models.
Furthermore, LCB58A demonstrated low cytotoxicity in normal cells (PBMCs and CD34+ HSCs) and exhibited a positive toxicokinetic (TK) profile.
"LCB58A addresses the limitations of existing 온라인 바카라 사이트 ADCs by exhibiting selective tumor cell killing. It also demonstrated superior anticancer efficacy in the presence of soluble 온라인 바카라 사이트 compared to tusamitamab," Chung remarked.
The company further emphasized the potential to broaden indications across various solid tumors and its commercial prospects. In addition to non-small cell lung cancer, the pipeline may be extended to include 온라인 바카라 사이트-expressing colorectal cancer.
"Although our primary target is lung cancer, representing a large market, we anticipate that confirmation of efficacy in colorectal cancer would establish this therapy as a highly promising ADC," Chung said. "We plan to initiate Phase 1 clinical trial in the second quarter of 2027," he added.

