- Emphasizing superior ‘drug release stability’ over existing treatments
- Strong interest from global pharmaceutical giants including Novo Nordisk, Amgen, Regeneron, and Madrigal Pharmaceuticals at ADA
- Partnering with Genentech, AbbVie, Sanofi, Roche, and LEO Pharma, including follow-up meetings with Novo held at BIO USA
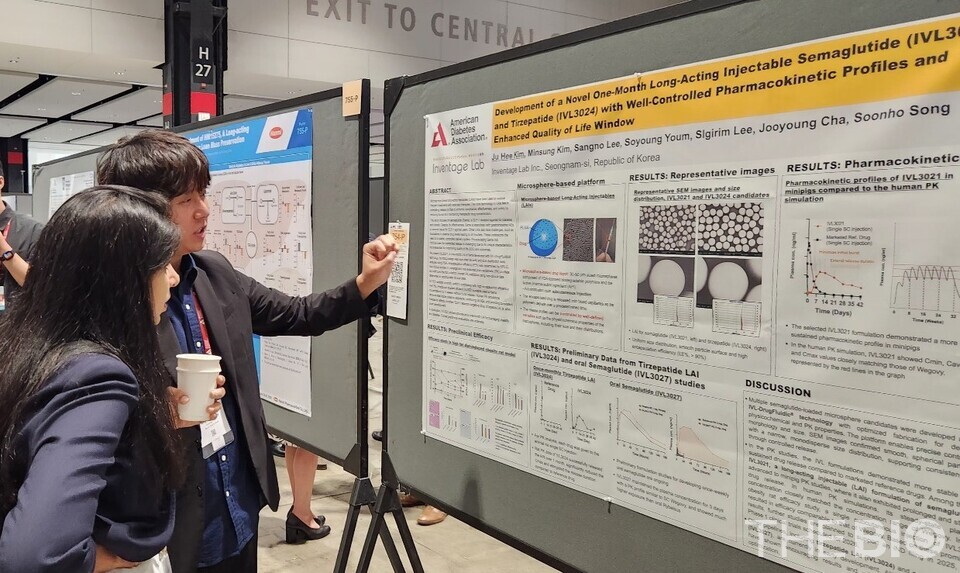
[by Kang, In Hyo] Inventage Lab, a company specializing in drug delivery technology platforms, announced on June 24 that it participated in the American Diabetes Association (ADA 2025) Annual Meeting, held in Chicago, USA, from June 20 to 23 (local time). During the conference, the company presented its next-generation obesity treatment platform technology in a poster session.
In this presentation, 스피드 바카라 Lab introduced preclinical data on its pipeline of long-acting and oral formulations (IVL3021 and IVL3024), a once-monthly injectable candidate based on semaglutide and tirzepatide, as well as an oral formulation of semaglutide (IVL3027).
According to the presentation, 스피드 바카라 Lab’s long-acting injection formulations, developed using the company’s proprietary ‘microsphere’ technology, demonstrated notable advantages such as excellent particle size uniformity and effective suppression of the initial burst release phenomenon, features that contribute to stable and sustained drug delivery. Additionally, the oral semaglutide candidate ‘IVL3027’ garnered significant interest from conference attendees due to its markedly improved bioavailability over existing oral formulations and its ability to maintain drug release for up to one week.
According to the company, numerous global pharmaceutical companies, including Novo Nordisk, Amgen, Regeneron, and Madrigal Pharmaceuticals, visited 스피드 바카라 Lab’s poster session and expressed strong interest. These companies highly regarded the absence of initial release in 스피드 바카라 Lab’s long-acting injectable formulations, recognizing it as a key feature that could enhance competitiveness in real-world clinical settings. Furthermore, 스피드 바카라 Lab’s long-acting injectable and oral drug delivery platforms were evaluated as promising solutions capable of addressing key limitations of existing glucagon-like peptide 1 (GLP-1) therapies, particularly in terms of patient compliance and high cost structure.
Building on the strong interest observed at both the 2025 ‘BIO USA,’ held last week in Boston, USA, and the ADA Annual Meeting, 스피드 바카라 Lab announced plans to actively pursue discussions on global technology transfer and co-development collaborations. Prior to participating in ADA, the company operated a standalone booth at BIO USA, where it conducted follow-up meetings with Novo Nordisk and held partnership discussions with Genentech, AbbVie, Sanofi, Roche, and Leo Pharma.
스피드 바카라 Lab intends to reinforce its strategic partnerships in key markets, including the United States and Europe, and to advance the global commercialization roadmap for its pipeline based on the company’s proprietary platform technologies, ‘IVL-DrugFluidic’ and ‘IVL-PePOFluidic.’

