IR session held on the afternoon of June 30 for shareholders, institutional investors, and press…Around 40 global pharmaceutical company officials visit company site

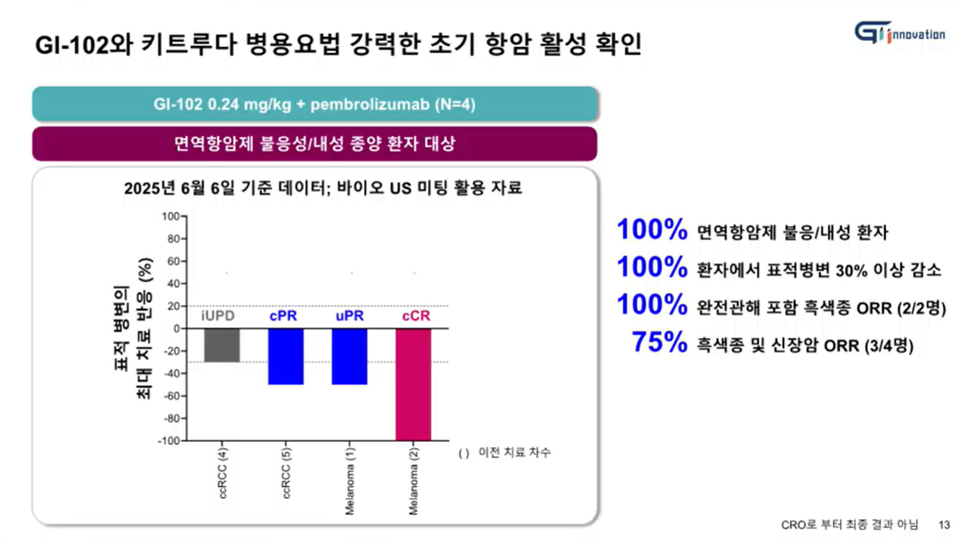
[by Ji, Yong Jun] GI Innovation has reported promising early clinical outcomes from the combination therapy of its immunotherapy candidate ‘GI-102 (development code)’ and MSD (Merck, USA)’s immune checkpoint inhibitor ‘Keytruda’ in patients who were refractory or resistant to prior immunotherapy. As of July 6, the objective response rate (ORR) for the ‘GI-102 + Keytruda’ combination therapy reached 75% (3 out of 4 patients), with one patient achieving complete remission (CR).
On the afternoon of June 30, GI Innovation conducted an online corporate briefing (IR) session for shareholders, institutional investors, and media press, during which it presented early data from the Phase 2 clinical trial of the ‘무료 바카라 게임102 + Keytruda’ combination therapy. Participating in the briefing were GI Innovation CEO Jang Myoung-ho, CEO Hong Jun-ho, and Clinical Dev & Strategy SVP Yun Nari. The session included updates on the development status of 무료 바카라 게임102, the company’s strategy for pursuing accelerated approval, an overview of its next-generation pipeline currently under development, and information related to ongoing technology licensing efforts.
◇무료 바카라 게임102 + Keytruda combination shows promising preliminary data in its early Phase 2 clinical trial
The early Phase 2 clinical data for the ‘무료 바카라 게임102 + Keytruda’ combination therapy was publicly disclosed for the first time during the briefing. GI Innovation expressed optimism regarding the results, noting that 3 out of 4 patients with melanoma or renal cancer, who are refractory or resistant to prior immunotherapy, achieved an ORR. In particular, in the melanoma subgroup, the ORR reached 100% (2 out of 2 patients), including one case of CR. Additionally, GI Innovation reported that in renal cancer patients not formally counted in the ORR metric, target lesions were nonetheless reduced by more than 30%, indicating that tumor responses were observed in the entire cohort of immunotherapy-resistant patients.
“Although these are preliminary results, we have observed early anticancer activity that is significantly stronger and more promising than what is typically seen with monotherapy,” SVP Yun emphasized. “This is a particularly encoura무료 바카라 게임ng development, 무료 바카라 게임ven the current lack of effective treatment for patients who are resistant or unresponsive to existing immunotherapies,” she added.
무료 바카라 게임102 is a bispecific Fc fusion protein composed of ‘CD80’ and ‘modified interleukin-2 (IL-2v3),’ designed to enhance immune cell activity within the tumor microenvironment. It exhibits potent ‘anticancer immune cell proliferation capacity,’ increasing immune cell counts by an average of 5 times. In monotherapy dose-escalation clinical trials, 무료 바카라 게임102 has demonstrated promising efficacy, with seven cases of confirmed partial remissions (PR) observed.
◇“Targeting FDA ‘Accelerated Approval’ of 무료 바카라 게임102 in 2028”
GI Innovation also presented its strategy for pursuing ‘Accelerated Approval’ of 무료 바카라 게임102 by the U.S. Food and Drug Administration (FDA), targeting the third quarter of 2028 as the anticipated approval timeline. The company noted that it is formulating its accelerated regulatory strategy by referencing the approval procedure of the U.S.-based company Replimune.
Replimune observed a meaningful therapeutic response in its clinical trial (IGNYTE), involving 156 melanoma patients who were refractory or resistant to existing PD-1 inhibitors. The study evaluated a combination therapy of Replimune’s tumor-derived oncolytic virus treatment ‘RP1 (vusolimogene oderparepvec)’ and MSD’s immune checkpoint inhibitor ‘nivolumab.’ Based on these results, Replimune is currently pursuing the FDA’s Accelerated Approval procedure for the corresponding indication.
According to GI Innovation, the global market for immune-oncology resistance is projected to grow from KRW 52 trillion (approximately USD 38.4 billion) in 2023 to about KRW 153 trillion by 2033. “We have already conducted an analysis, in collaboration with consulting firms and Dr. Yoo Tae-ho, to assess the feasibility of obtaining accelerated FDA approval,” Yun stated. “무료 바카라 게임102 is poised to become the first immuno-oncology drug developed in Korea to secure accelerated FDA approval.”
◇Accelerated development of PD-1·VEGF+‘α’ ‘triple antibody’
무료 바카라 게임 Innovation has also initiated the development of triple antibody therapeutics. This follows recent advancements such as ‘ivonescimab,’ a PD-1·VEGF dual antibody candidate co-developed by China’s Akeso Biopharma and the U.S.-based Summit Therapeutics, which has introduced a novel treatment approach in the immuno-oncology market. Building on this momentum, 무료 바카라 게임 Innovation plans to retain the two PD-1 and VEGF targets of dual antibodies while enhancing the tumor-targeting capability of a third antibody.
“Our VEGF-targeting antibodies have been developed to a level that matches, if not exceeds, the performance of existing therapies currently on the market or in development,” CEO Jang Myoung-ho explained.
◇40 executives from global pharmaceutical companies visit company… “Targeting technology transfer deal within the year”
Global technology transfer negotiations for 무료 바카라 게임102 are reportedly proceeding smoothly. Recently, around 40 executives from major global pharmaceutical companies visited GI Innovation to conduct an on-site due diligence inspection of 무료 바카라 게임102’s clinical development status, toxicity data, chemistry, manufacturing, and controls (CMC), intellectual property, and other related fields.
“Is it highly unusual for global pharmaceutical companies to carry out technology evaluations involving more than 40 vice presidents and team leaders,” Jang remarked. “Internally, we interpret this level of engagement as a strong ‘green light’ for potential technology export.”
Jang has set a goal of securing a technology licensing deal for 무료 바카라 게임102 within the year. He emphasized that the broad patent protection for 무료 바카라 게임102, which extends until 2045, provides a significant strategic advantage in negotiations with global pharmaceutical companies targeting the immunotherapy resistance market.
“For 무료 바카라 게임102, we are open to both indication-specific technology licensing and full asset transfer,” Jang said. “As we place a high priority on shareholder interests, any proposal exceeding KRW 10 trillion will be subject to serious internal consideration,” he added.

