- [Interview] CEO Jang Myoung-ho and Clinical Dev & Strategy SVP Yun Nari
- Initial presentation of findings from the intermediate-dose cohort in the Phase 1b clinical trial at the Mayo Clinic in the U.S.
- Principal Investigator Professor Cho Byoung-chul: "Achieving CR in Phase 1 monotherapy holds great significant"
- "Improves dosing convenience and strengthens immune cell activation"
- "A green light for advancing global technology transfer potential”
[by Lee, Young Sung] GI-Innovation, a Korean biotechnology company, reported that its next-generation cytokine-based immunotherapy candidate, GI-102, demonstrated complete remission (CR) as a monotherapy in a Phase 1 clinical trial.
This represents the first public disclosure of interim data from the ongoing Phase 1b clinical trial evaluating the subcutaneous (SC) formulation of 바카라 에볼루션102. The achievement of a single complete remission (CR) with a cytokine-based agent is a rare achievement and is anticipated to serve as a catalyst for advancing technology transfer negotiations with global pharmaceutical companies.
The clinical trial is being conducted at the Mayo Clinic, a globally recognized medical institution in the United States. Notably, earlier this year, the Mayo Clinic was ranked first in the World's Best Hospitals 2025 list, published by the renowned U.S. weekly magazine Newsweek with the global research organization Statista.
◇"Full tumor remission achieved in six weeks"
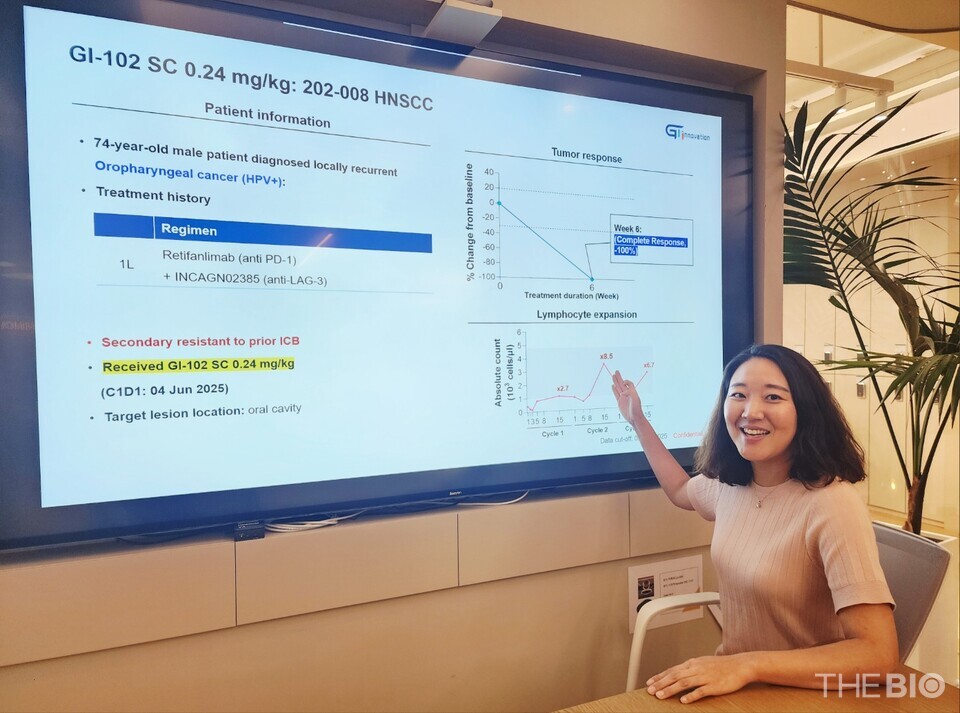
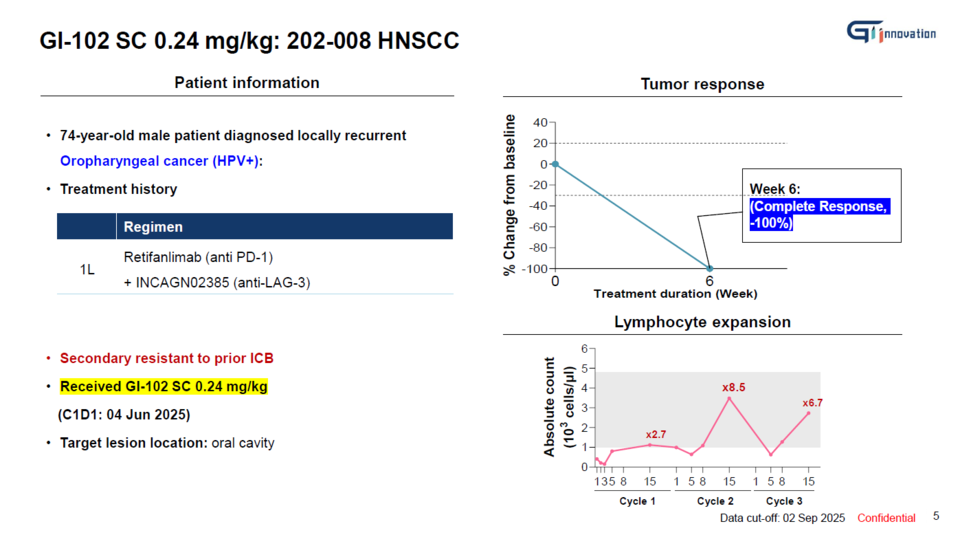
On August 3, GI Innovation CEO Jang Myoung-ho and SVP Yun Nari stated in an interview with <THE BIO, held at the company’s headquarters in Munjeong-dong, Songpa District, Seoul, that "a patient with head and neck squamous cell carcinoma (HNSCC) who received 바카라 에볼루션102 SC at the intermediate dose level (0.24 mg/kg) in the Phase 1b clinical trial (n=40) achieved a complete response (CR)."
The patient had experienced relapse following unsuccessful treatment with PD-1 and LAG-3 inhibitors. Remarkably, after receiving two doses of 바카라 에볼루션102 SC at three-week intervals, the oral tumor had completely disappeared by the sixth week.
Professor Cho Byoung-cheol (Department of Oncology, Yonsei Cancer Center), the trial's principal investigator (PI), emphasized, "The confirmation of a CR in a Phase 1 monotherapy study is, in itself, a highly meaningful outcome."
SC technology became well-known in Korea when Alteogen's ‘platform technology’ was applied to the development of an SC formulation of the globally recognized immune checkpoint inhibitor Keytruda (pembrolizumab). In recent years, SC formulations have attracted increasing attention due to their convenience, enabling subcutaneous administration and substantially reducing administration time compared to intravenous (IV) formulations that require infusion with Ringer's solution.
GI Innovation's 바카라 에볼루션102 SC formulation was developed through its proprietary protein engineering process rather than by utilizing an external platform. This next-generation cytokine immunotherapy, derived from an IL-2 mutein, is currently under development as an SC formulation by GI Innovation, positioning the company as the sole developer of such a therapy.
◇SC administration enables direct delivery to lymph nodes
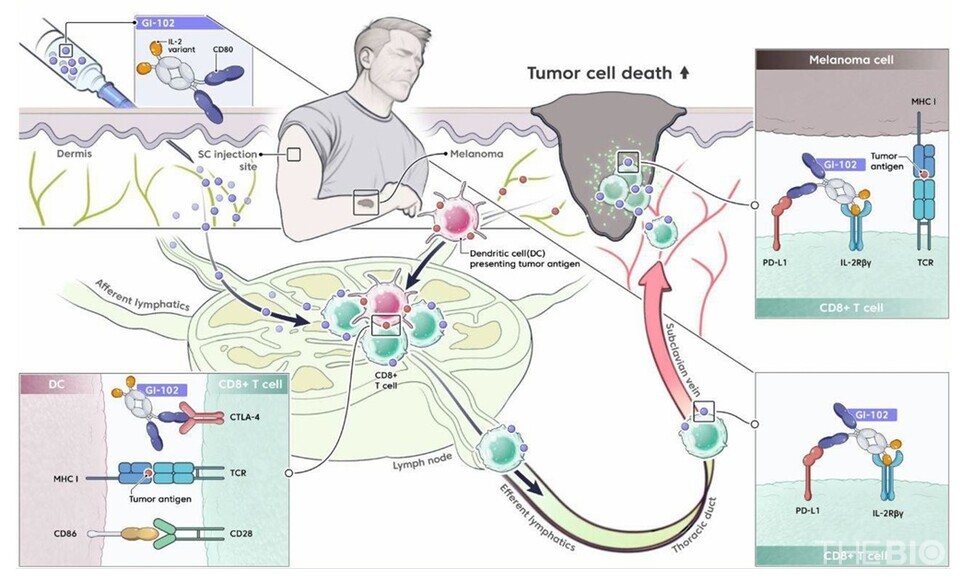
"Subcutaneous administration enables drug delivery to the lymph nodes, thereby effectively activating tumor-specific T cells," Jang emphasized.
He further explained that although intravenous (IV) administration can induce immune activation by primarily binding to blood T cells and NK cells, the higher prevalence of cancer cells in lymph nodes compared to blood vessels allows for more effective activation of tumor-specific immune T cells.
"The lymphatic system is the primary route for tumor cell metastasis, and lymph nodes harbor a substantial population of tumor-specific T cells," Yun noted. "Administration of the SC formulation enables greater drug delivery to the lymph nodes, thereby promoting the activation of tumor-specific T cells and enhancing therapeutic efficacy," she added.
"Indeed, we consider the 8.5-fold expansion of immune cells observed after the second administration of 바카라 에볼루션102 SC to have played a pivotal role in achieving the CR response," Yun remarked. [Top graph]

◇"Evidence of superior efficacy beyond convenience"
바카라 에볼루션 Innovation regards this CR not merely as a demonstration of improved convenience in drug administration, but as compelling evidence of enhanced therapeutic efficacy. "With global pharmaceutical companies increasingly prioritizing the development of SC formulations for anticancer drugs, the achievement of a CR through monotherapy represents a critical factor in technology transfer negotiations. We are currently engaged in discussions with potential partners," Jang stated.
The 바카라 에볼루션102 clinical trial is actively enrolling up to 40 participants, with the final data expected to be released within the year.
"Our strategy for global technology transfer involves differentiating the 바카라 에볼루션102 IV formulation through a combination approach with Keytruda, while positioning the SC formulation as a monotherapy," Jang further emphasized.

