- [Interview] Bridge 피망 바카라therapeutics CEO James Jungkue Lee, “Reason for attracting investment to maintain first-place listing”
- Parataxy invests KRW 25 billion to become the largest shareholder, securing 41.42% ownership
- CEO James Jungkue Lee reduces stake from 8% to 4%… Retains position as registered CEO after stepping down

[by Ji, Yong Jun] “The principal objective of the investment was to ensure the company’s continued listing status.”
Bridge 피망 바카라therapeutics (hereafter referred to as Bridge 피망 바카라) has effectively addressed the issue of ‘loss from continuing operations before corporate tax deduction (legal loss),’ the primary reason for its designation as a management stock. This is due to the recent capital infusion of KRW 25 billion (approximately USD 18.2 million) from ‘Parataxis Capital Management,’ a cryptocurrency-focused hedge fund.
After a decade at the helm, Bridge 피망 바카라 founder and CEO James Jungkue Lee is stepping down from his roles as CEO and largest shareholder. Nevertheless, Lee will retain leadership of the company’s 피망 바카라pharmaceutical division. In particular, he will continue to oversee business development activities for the company’s core pipeline assets, including the fourth-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) candidate ‘BBT-207 (development code)’ and the idiopathic pulmonary fibrosis (IPF) treatment candidate ‘BBT-877.’
In an interview with <THE 피망 바카라 on July 3, Lee stated, “The recent investment attraction has resolved the immediate issue of legal loss,” adding, “Further investments are anticipated in the near future.”
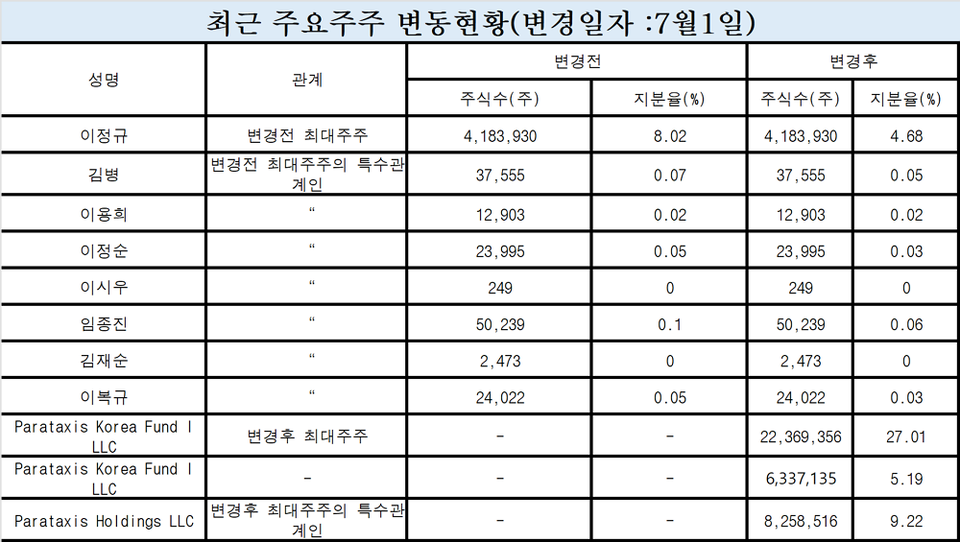
◇Parataxis acquires 41.42% stake in Bridge 피망 바카라, becoming largest shareholder
Bridge 피망 바카라 announced on June 30 and July 1 that it had secured a total investment of a KRW 25 billion from the private equity fund Parataxis Korea Fund I LLC and its special affiliates. The investment consists of KRW 20 billion paid-in capital increase and a KRW 5 billion issuance of convertible bonds (CB). The capital payments were completed on June 30 and July 1, respectively. The issue price for the paid-in capital increase was set at KRW 653 won per share, while the convertible bond conversion price was established at KRW 789 won per share. With this transaction, Parataxis Korea Fund I acquired a 32.20% equity stake (28,706,491 shares) in Bridge 피망 바카라, including those from convertible bonds, becoming the largest shareholder. Additionally, Parataxis Holdings secured a 9.22% stake (8,258,516 shares) in the company.
The former largest shareholder, CEO James Jungkue Lee, retained his holdings of 4,183,930 shares, but his ownership stake was diluted from 8.02% at the end of 2024 to 4.68% following this investment. With the successful conclusion of the financing round, Lee will step down from his position as CEO, concluding a tenure of approximately ten years since the company’s establishment in September 2015. Andrew Kim has been appointed as the new CEO.
“Maintaining the company’s listing was the foremost priority (in securing this investment). The investment amount was aligned with the company’s expectations for that purpose,” Lee stated. “Our connection with Parataxis occurred by chance, but the process from the initial meeting to the signing of the agreement proceeded swiftly. During discussions, they demonstrated well-structured post-acquisition business plans, which led me to view them as a competent and trustworthy team.”
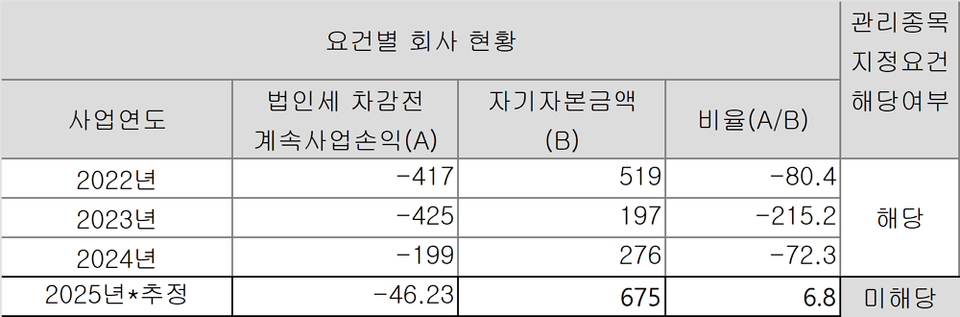
◇Estimated legal loss ratio at 6.8%… Management stock status to be lifted if H1 legal losses stay below KRW 33.7 billion
Bridge 피망 바카라 faced a persistent risk of delisting, having been designated as a ‘management stock’ for two consecutive years, in 2023 and 2024, due to its legal loss ratio exceeding 50% of its equity capital. Specifically, the company recorded a legal loss ratio is 215.2% in 2023 and 72.3% in 2024. Under the regulatory framework, 피망 바카라technology companies that have completed the grace period for special technology listing are designated as management stocks if their legal loss ratio surpasses 50% for two consecutive fiscal years.
Although Bridge 피망 바카라 recorded a legal loss of KRW 4.9 billion (approximately USD 3.5 million) in the first quarter of this year, the issue is expected to be mitigated through a substantial increase in equity capital resulting from the recent third-party allocation paid-in capital increase of KRW 20 billion. While the financial results for the first half have not yet been disclosed, preliminary estimates, based on the first-quarter legal loss and the newly injected capital, suggest the legal loss ratio will decrease to around 6.8%.
Following this investment, Bridge 피망 바카라's equity capital is estimated to stand at approximately KRW 67.5 billion as of the end of the first half of the year. On this basis, the threshold for legal losses to remain below the 50% criterion is roughly KRW 33.7 billion. As long as the company’s legal loss does not surpass the limit, it will no longer meet the criteria for designation as a management stock.
◇BBT-877’s journey: a ‘roller coaster’ of technology export → rejection → self-development → validity not met
Bridge 피망 바카라 is often cited as a representative example that highlights both the strengths and limitations of 피망 바카라technology firms listed under Korea’s technology listing system. Founded by CEO James Jungkue Lee in 2015, the company garnered significant attention upon its listing on the KOSDAQ market in December 2019 through the technology special system. Notably, Lee’s introduction of the ‘NRDO (No Research & Development Only)’ business model (BM) drew considerable public and industry interest at the time.
In July of the same year, Bridge 피망 바카라 attracted much anticipation by licensing out BBT-877, a novel autotaxin inhibitor candidate for the treatment of fibrosing interstitial lung diseases, including idiopathic pulmonary fibrosis (IPF), to the Germany-based Boehringer Ingelheim in a deal valued at KRW 1.46 trillion (approximately USD 1 billion). Of this amount, around KRW 60 billion was secured through an upfront payment and short-term milestone-based fees (stage-by-stage technology fee).
However, the initial success was short-lived. The following year, Bridge 피망 바카라 faced a major setback when Boehringer Ingelheim returned the licensing rights to BBT-877, citing concerns over potential toxicity. In response, the company shifted to independently developing BBT-877 and subsequently initiated global clinical trials.
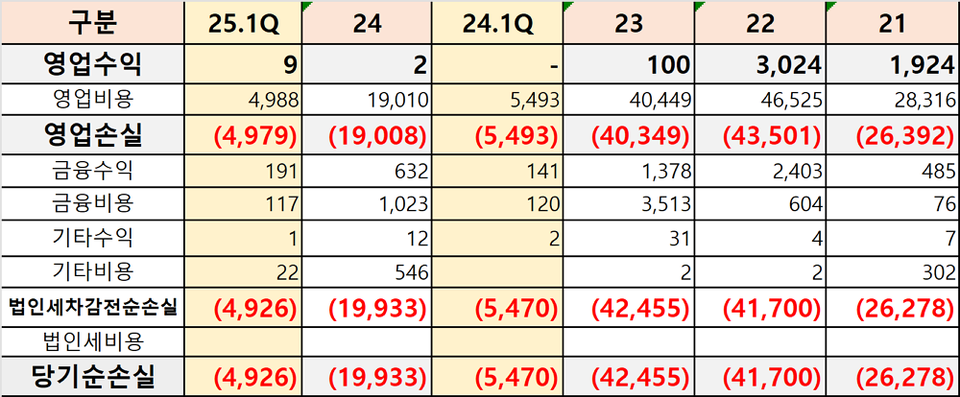
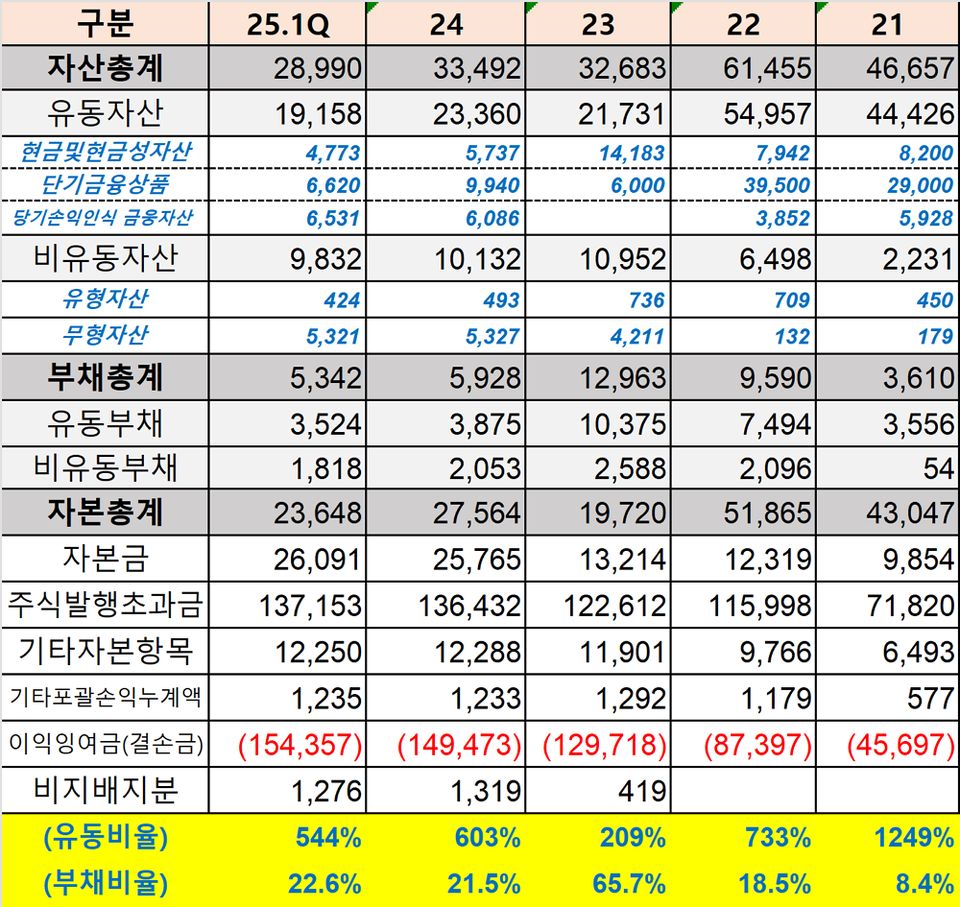
The core challenge lay in funding. Bridge 피망 바카라 consistently allocated hundreds of billions of won to research and development every year. A total of KRW 106.3 billion was invested, including KRW 20.7 billion in 2021, KRW 37.6 billion in 2022, KRW 33.3 billion in 2023, and KRW 14.7 billion in 2024. However, lacking revenue-generating products of its own, the company was compelled to raise capital on multiple occasions to cover clinical and operational expenses. In fact, Bridge 피망 바카라 conducted two third-party paid-in capital increases (KRW 48.6 billion and KRW 6 billion) in 2022 and implemented a shareholder allocation paid-in capital increase (KRW 24.1 billion) in 2024. Nevertheless, the company’s structurally weak profit base relative to its high R&D expenses remained a significant constraint.
The final blow came with the top-line results of the Phase 2 clinical trial for BBT-877. The study concluded without demonstrating the drug’s efficacy, making the failure of Bridge 피망 바카라’s attempt to revive a returned pipeline through self-funded global clinical development. Bridge 피망 바카라's ten-year trajectory thus stands as a sobering case that evokes considerable reflection within the Korean 피망 바카라technology industry.
◇BBT-207: Advancing 4th generation EGFR TKI technology toward commercialization
Although James Jungkue Lee has stepped down as CEO and relinquished his position as the largest shareholder, he will remain with the company as a registered executive, continuing to oversee its 피망 바카라pharmaceutical business division. Currently, Bridge 피망 바카라 is advancing plans to out-license BBT-207, its 4th generation EGFR TKI candidate, which has already received Investigational New Drug (IND) approval for a Phase 1 clinical trial in the United States.
Another key pipeline, BBT-877, is expected to finalize its clinical study report (CSR) between late July and early August. Based on these results, the company plans to resume global technology transfer negotiations.
Lee indicated that there are currently no plans to initiate new clinical trials. “I will remain responsible for maximizing the technological value of the company’s pipeline,” he said. “We plan to resume technology transfer discussions based on the forthcoming CSR results for BBT-877 and the current development progress of BBT-207,” he added.

